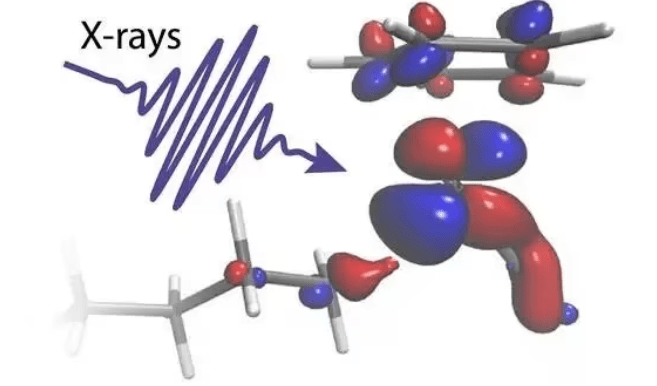
An X-ray flash illuminates a molecule. Credit: Raphael Jay
Topics: Chemistry, Climate Change, Green Tech, High Energy Physics, Research, X-rays
The use of short flashes of X-ray light brings scientists one big step closer to developing better catalysts to transform the greenhouse gas methane into a less harmful chemical. The result, published in the journal Science, reveals for the first time how carbon-hydrogen bonds of alkanes break and how the catalyst works in this reaction.
Methane, one of the most potent greenhouse gases, is being released into the atmosphere at an increasing rate by livestock farming as well as the continuing unfreezing of permafrost. Transforming methane and longer-chain alkanes into less harmful and, in fact, useful chemicals would remove the associated threats and, in turn, make a huge feedstock for the chemical industry available. However, transforming methane necessitates, as a first step, the breaking of a C-H bond, one of the strongest chemical linkages in nature.
Forty years ago, molecular metal catalysts were discovered that can easily split C-H bonds. The only thing found to be necessary was a short flash of visible light to “switch on” the catalyst, and, as by magic, the strong C-H bonds of alkanes passing nearby are easily broken almost without using any energy. Despite the importance of this so-called C-H activation reaction, it remained unknown over the decades how that catalyst performs this function.
The research was led by scientists from Uppsala University in collaboration with the Paul Scherrer Institute in Switzerland, Stockholm University, Hamburg University, and the European XFEL in Germany. For the first time, the scientists were able to directly watch the catalyst at work and reveal how it breaks those C-H bonds.
In two experiments conducted at the Paul Scherrer Institute in Switzerland, the researchers were able to follow the delicate exchange of electrons between a rhodium catalyst and an octane C-H group as it gets broken. Using two of the most powerful sources of X-ray flashes in the world, the X-ray laser SwissFEL and the X-ray synchrotron Swiss Light Source, the reaction could be followed all the way from the beginning to the end. The measurements revealed the initial light-induced activation of the catalyst within 400 femtoseconds (0.0000000000004 seconds) to the final C-H bond breaking after 14 nanoseconds (0.000000014 seconds).
X-rays visualize how one of nature’s strongest bonds breaks, Uppsala University, Phys.org.